L A W S
Our standards:

REGULATION EU 2016/425 – PERSONAL PROTECTIVE EQUIPMENT (PPE)
PPE must be designed and manufactured so that the user can enjoy appropriate protection, in conformity with the instructions of the manufacturer, without suffering harm.
Regulation (EU) 2016/425, entered into force as of April 2018, is the reference standard for PPE. It classifies equipment in the following categories:
Class Ia
Minimal or minor risks (CE marking). Simple design PPE PPE is subject to a self-certification procedure by which the manufacturer must check conformity with the technical standards and the essential requirements concerning safety and health
Class IIa
PPE that do not fall within Class Ia and IIIa (CE marking + risk pictogram). PPE must be certified by a Certification Body recognised at European level. It must pass a number of tests and checks aimed at verifying that it meets the requirements contemplated in the reference standards. The manufacturer is responsible for surveillance of Category IIa PPE in order to maintain the protection standards of the PPE over time.
Class IIIa
Risk of death or serious permanent injuries (CE marking + risk pictogram + number of Certification Body). Complex design PPE PPE requires that Certification Bodies issue a certificate of conformity and carry out regular inspections of production processes at the manufacturer’s premises. The obligation of PPE surveillance lies exclusively with the Certification Body that has issued the certificate where the PPE has been certified as Category IIIa.

GENERAL REQUIREMENTS FOR PROTECTIVE GLOVES EN 420:2003+A1:2009
Standard EN 420:2003+A1:2009 (see table 1) defines essential requirements such as:
• Harmless (gloves should be harmless for the wearer: the content in hexavalent chromium should be checked along with the pH level; for natural rubber gloves the level of proteins in latex should be checked).
• Construction (max. protection and performance optimisation).
• Compliance with standard dimensions, excluding special design gloves (Table 1).
• Instructions for use.
• Compliance with the marking instructions.
TABLE 1 | Standard glove dimensions


MECHANICAL RISKS EN 388:2016
Standard EN 388 (see table 2), as lastly revised in 2016, is the product standard that defines the features, types and protection levels against mechanical risks.
The protection guaranteed is as follows:
– Abrasion resistance (specific resistance of a glove to continuous use on rough surfaces);
– Blade cutting resistance (protection index against sharp objects);
– Tear resistance (protection index against risks of tearing);
– Perforation resistance (understood as a barrier against pointed objects which could perforate the glove);
– TDM cutting resistance (test used to assess the cutting resistance considering the resistance of the blade and the orthogonal pressure exerted on the blade);
– Impact resistance (optional)..
TABLE 2 | Mechanical risks


PROTECTION AGAINST DANGEROUS CHEMICALS AND MICRO-ORGANISMS EN ISO 374:2016
Standard EN ISO 374:2016 defines the requirements for protective gloves against chemical and/or microbiological risks
CHEMICAL RISK EN ISO 374-1:2016
This section of the standard defines the protection requirements and methods concerning gloves designed to protect the wearer against chemical risks. Below is a list of the reference tests:
• Penetration test (test method pursuant to EN ISO 374-2:2014): penetration means the transfer, at non molecular level, of a chemical or a micro-organism through the pores, seams and micro-holes or imperfections of a material.
• Permeation tests (pursuant to standard EN 16523-1:2015, superseding standard EN 374-3:2003): permeation means the process by which a chemical product spreads at molecular level through the glove material (see table 5).
• Degradation test (EN 374-4:2013): to check the alteration of the physical properties of a glove (swelling, change in dimensions, bulging, etc.) upon contact with a chemical for a predefined period of time.
Gloves are classified in three different types. These are marked differently (Table 3) according to the number of substances tested, as updated after the introduction of 6 new chemicals (highlighted in Table 4), and the migration time (Table 5).
TABLE 3 | Type identification
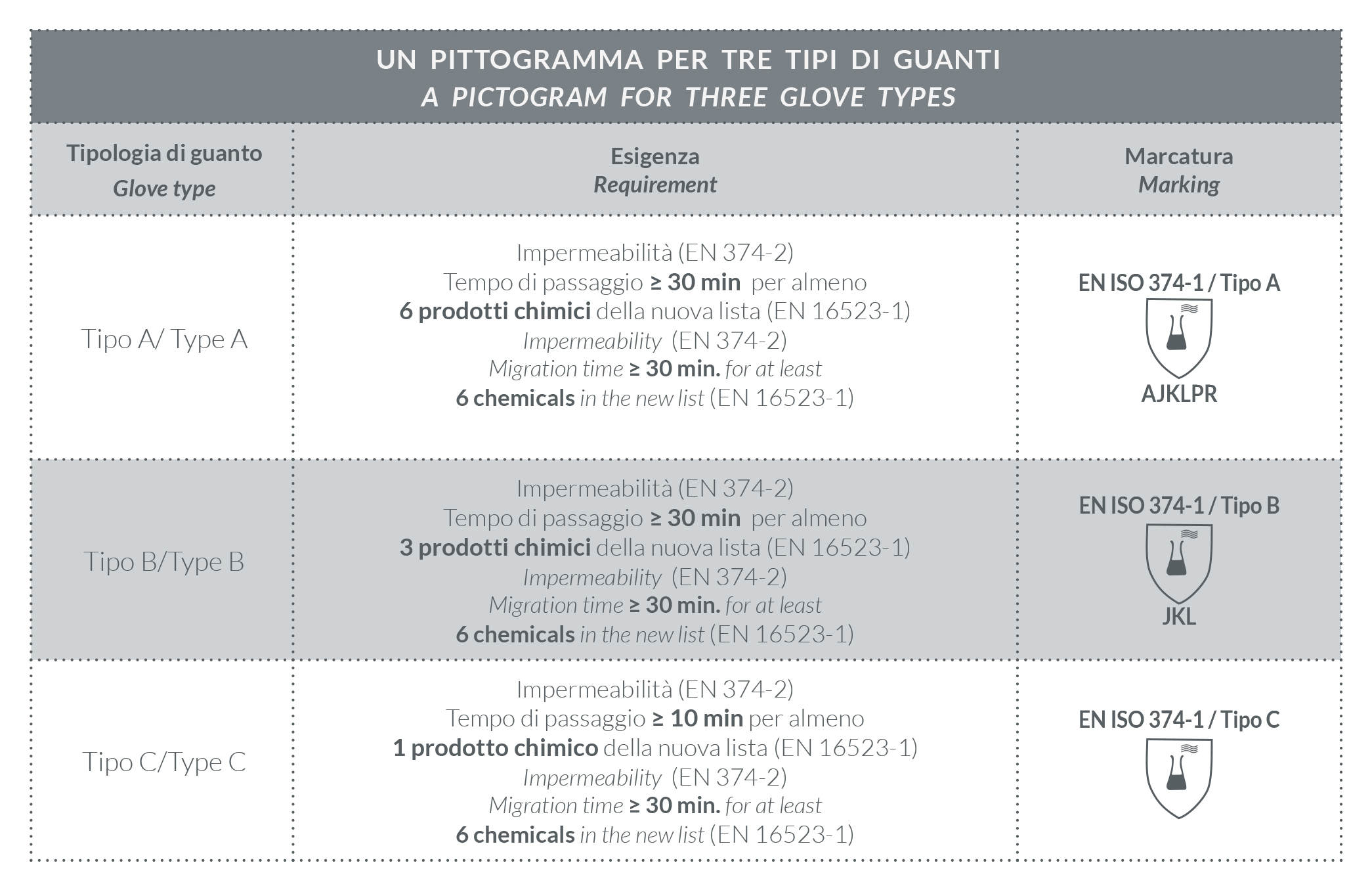
TABLE 4 | Protection from chemical agents
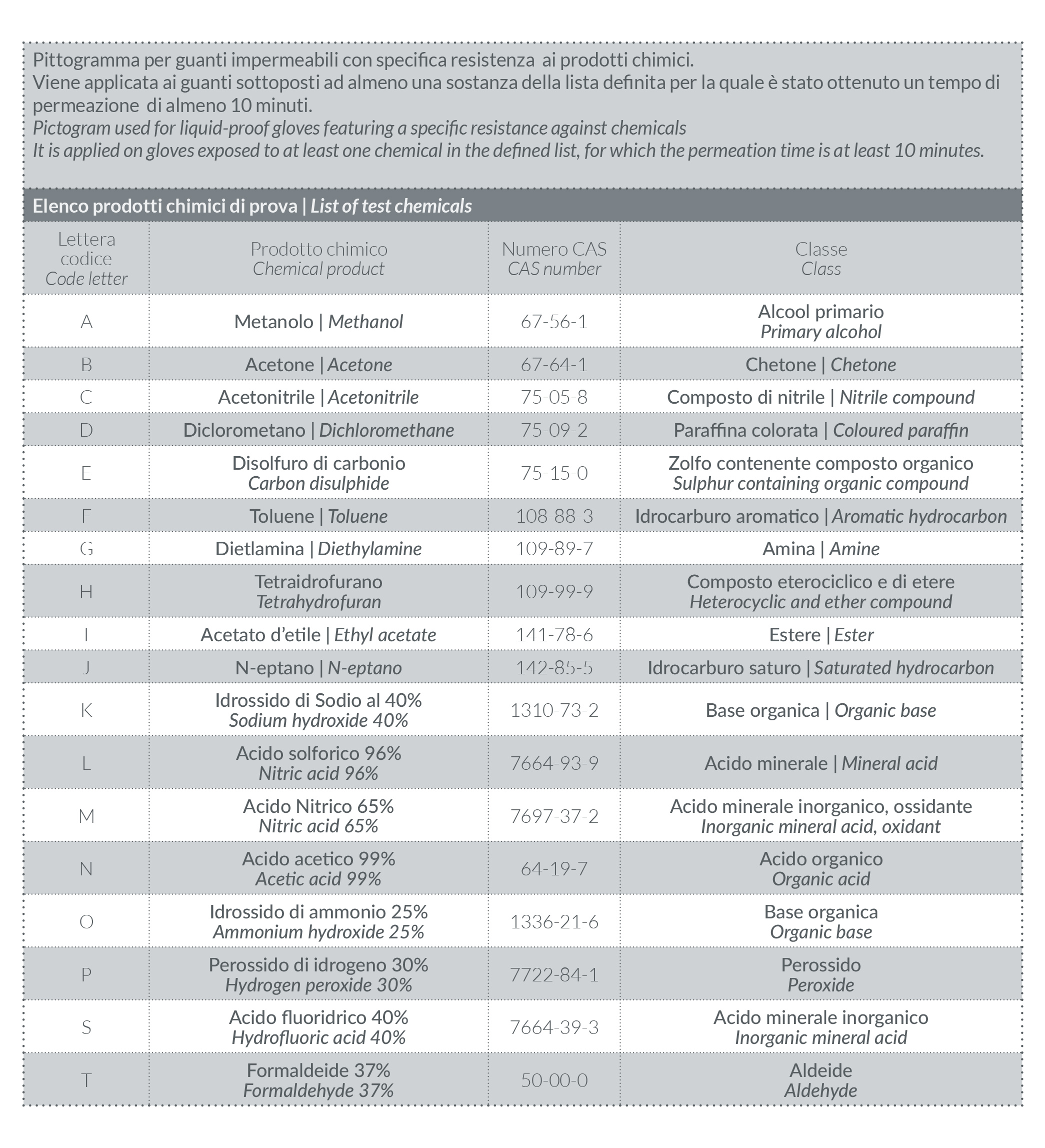
TABLE 5 | Performance indexes of permeation times


PROTECTIVE GLOVES AGAINST MICRO-ORGANISMS EN ISO 374-5:2016
This section of the standard defines the protection requirements and methods concerning gloves designed to protect the wearer against micro-organisms (see table 6).
Below is a list of the reference tests:
• Penetration test (test method pursuant to EN ISO 374-2:2014): penetration means the transfer, at non molecular level, of a chemical or a micro-organism through the pores, seams and micro-holes or imperfections of a material.
• Virus test (ISO 16604:2004/Method B): to check the protection ensured by the glove upon contact with pathogens contained in blood and body fluids (test method Phi-X 174).
TABLE 6 | Protection against micro-organisms


REGULATION EU 2017/745 – MEDICAL DEVICES
Italian Legislative Decree Regulation EU 2017/745, which abridge the European Directive 93/42/CEE, defines a medical device as any instrument, apparatus, appliance, material or other article, whether used alone or in combination, including the software necessary for its proper application intended by the manufacturer to be used for human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease; diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap; investigation, replacement or modification of the anatomy or of a physiological process; control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
MDs are classified in four classes: CLASS I, CLASS IIa, CLASS IIb and CLASS III depending on the features and the risk level associated to the medical device.
Based on the MD class, reference must be made to the relevant annex that provides a definition of the procedure to be followed for conformity assessment.
• CLASS I – Annex VII
• CLASS IIa – Annex VII+IV
• CLASS IIb – Annex II (excl. Point 4)
• CLASS III – Annex II (complete)
As regards CLASS I devices, no intervention is required by a Notified Body for the assessment of conformity, excluding CLASS I STERILE devices and CLASS I devices with a MEASUREMENT FUNCTION.
All other CLASSES require intervention by a Notified Body that is in charge of performing the specific activities contemplated in the relevant ANNEX.

MEDICAL GLOVES FOR SINGLE USE UNI EN 455
This standard disciplines the necessary requirements for a disposable glove, designed for use in the medical field, to fulfil its function of preventing and directly protecting both the patient and the wearer against cross-contamination.
This standard consists of four parts:
• EN 455-1:2002 – Freedom from holes. Requirements and testing. This part specifies the requirements and illustrates the method to be used for testing medical gloves for single use with a view to ascertaining freedom from holes (water tightness test for the identification of holes, sampling, control level and AQL).
• EN 455-2:2015 – Requirements and testing for physical properties. This standard specifies the requirements and illustrates the methods for testing the physical properties of medical gloves for single use (dimensions and resistance) in order to provide an adequate level of protection for both the patient and the wearer against mutual contamination during use.
• EN 455-3:2015 – Requirements and testing for biological evaluation. This standard specified the requirements relating to the evaluation of the biological safety of medical gloves for single use. It specifies requirements relating to labelling and packaging of gloves, and the dissemination of information concerning the test methods adopted.
• EN 455-4:2009 – Requirements and testing for shelf life determination. This standard specifies the requirements concerning the shelf life of medical gloves for single use. It also describes the requirements for labelling and the dissemination of information concerning the test methods adopted.

FOOD CONTACT MATERIALS
Any Material and Object intended for Food Contact shall be thoroughly inspected in order to prevent the risk of contamination and to ensure conformity with the requirements concerning health and safety.
The reference standards are: Regulation (EC) 1935/2004, Decree of the Italian Ministry for Health 21/03/1973 (natural or synthetic rubber gloves – see table 7), Regulation (EU) 10/2011 (gloves made with plastics – see table 8).
The device to be used should be selected after evaluating its suitability for contact with a specific foodstuff in real conditions of use, in other words the possible migration of substances between the foodstuff and the device should be checked depending on the following points:
• Quantities that constitute a health hazard
• Alteration of the food composition
• Alteration of the organoleptic properties of the food
These are the principles listed in Regulation EC 1935/2004 and are valid for all materials.
Compliance with these principles is checked by migration tests, where the term “migration” means the transfer (passage) of substances from the material to food.
Overall migration
Overall migration tests are used to determine the total quantity of the migrated substance without identifying the migrated compound or compounds. These are not specific tests. Tests are performed on food contact materials before food contact. This requires constant use of simulants, i.e. fluids that reproduce the features of the foodstuff connected to its extraction capacity
Specific migration
Specific migration tests are used to identify the migrating substance and its quantity
Tables of simulants used in migration testing
TABLE 7 | Ministerial Decree of 21/3/73

TABLE 8 | EU Regulation 10/2011

The foods indicated in the tables are purely indicative as the same food could be tested with different simulants according to the characteristics of the food.

Guidance to European standards and for selection
The EU has developed six “types” of garments providing protection against chemical risks
The certification of a specific type provides an indication of the protection of the garment against a specific type of risk (gas, liquid or dust – see table 9).
Additional protection rules obtained from the range of products are given in table 10.
TABLE 9 | Types of chemical protection garments

TABLE 10 | Protection standards


